Background
Consent is an absolutely crucial part of any study. Participants must be informed of the nature of the study, its purpose and any inherent risks or potential benefits. Studies which come under the definition of research will need some form of consent. If you need Trialflare to handle this - great! - we make this very simple for you to do.
💡 IMPORTANT: The contents of your informed consent form (ICF) should have been independently reviewed by an appropriate ethics committee, for example, a REC or IRB.
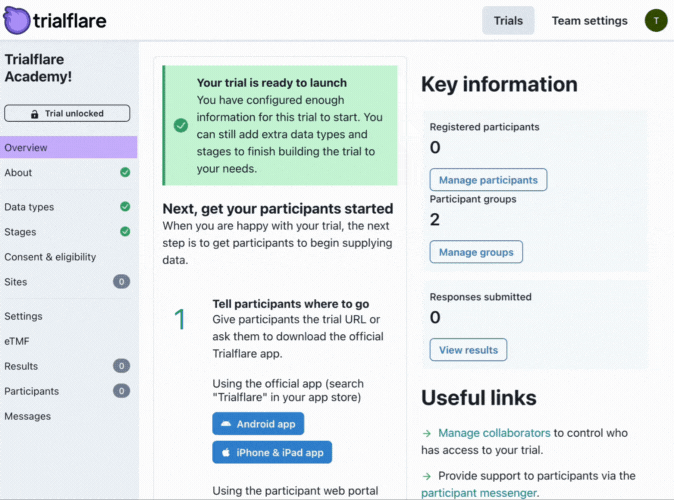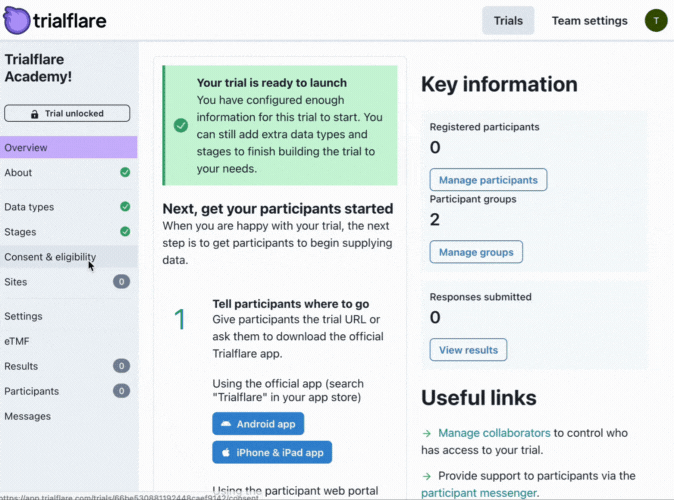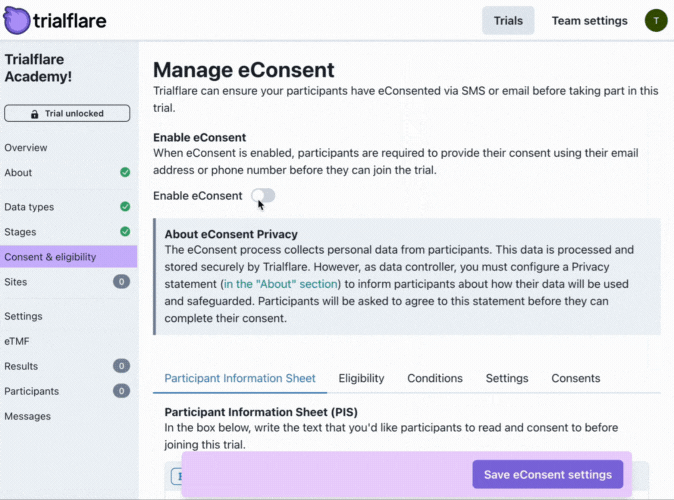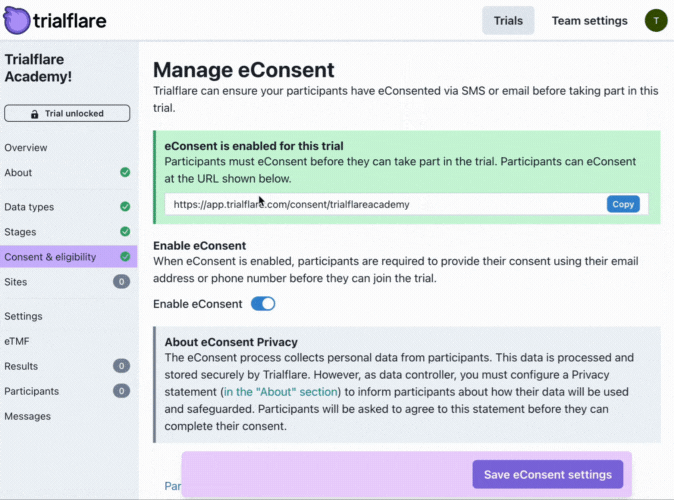
Enabling eConsent in your study
Making a study require consent is easy. Just check the tickbox to begin creating your form. There are multiple steps items to fill in when completing your consent form.
Participant Information Sheet (PIS)
What do you need the participant to know? PIS can take many shapes and sizes but background to the study, the purpose and overview and any potential risks/benefits are the key things to get started with.
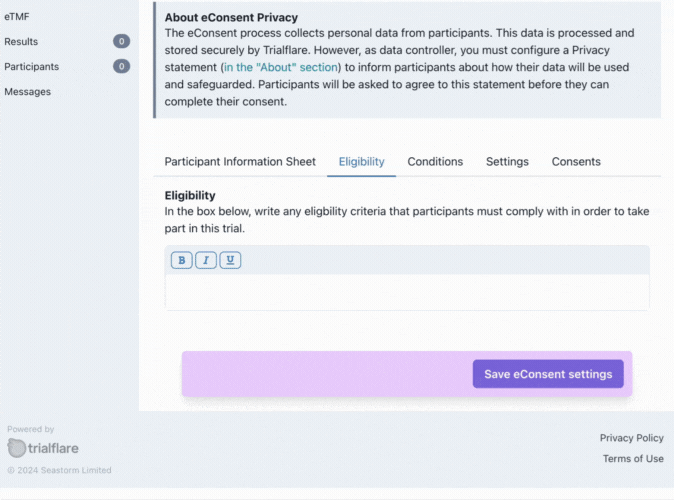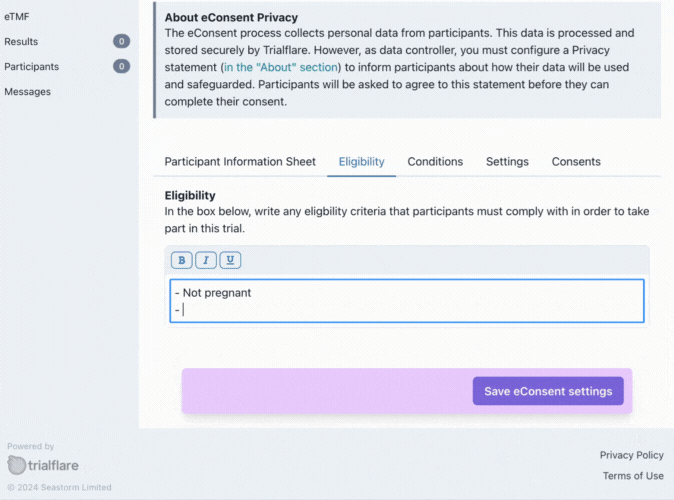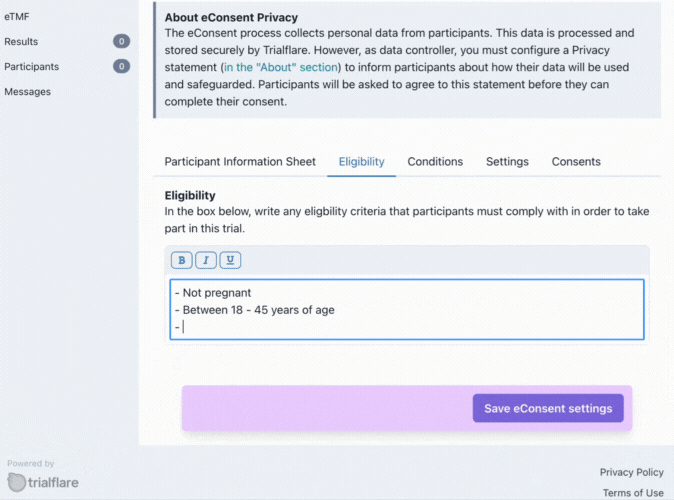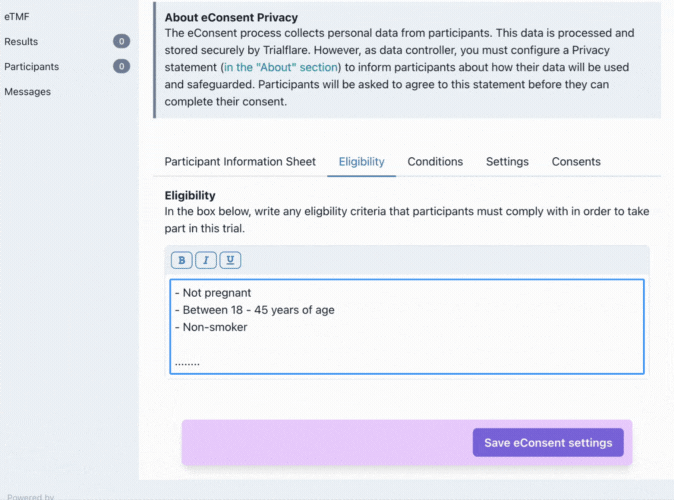
Eligibilty
What is your target audience? You can specify this here. These eligibility criteria will need to be initialled in the consent form for confirmation by the participant.
Conditions
Stand-alone conditions which must be initialed and checked are helpful in getting key information across to your participant. Are they aware of the risks? Are they aware that they can withdraw from the study at any time?
Settings
Trialflare stores all logs of eConsent for each study, however, you might want a particular member of staff to receive these by email.
💡 IMPORTANT: You will notice that the post-consent information which goes out to participants has a character limit. This is to keep your content within the constraints of SMS. This information/statement will go out to the same phone number the participant consented with, and, to their email address if they provided it also.
eConsents:
-
Will be sent to the participants for reference so they know what they have agreed to.
-
Will be stored on Trialflare for study team members to view or download.
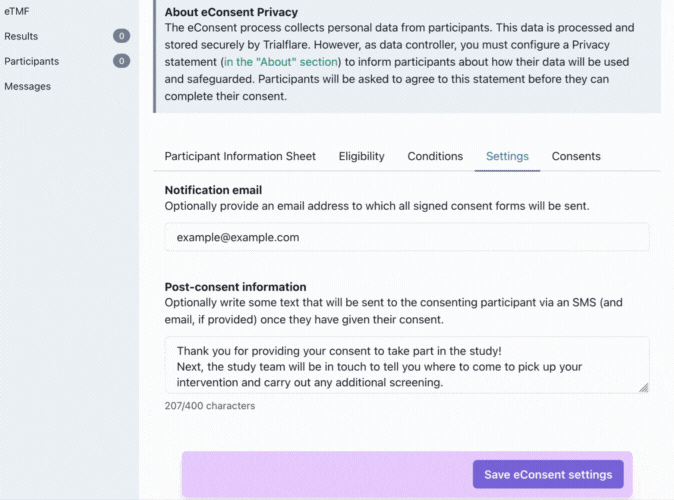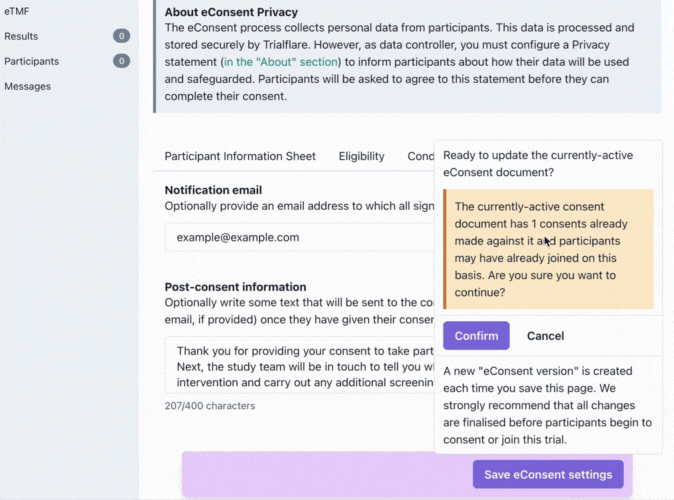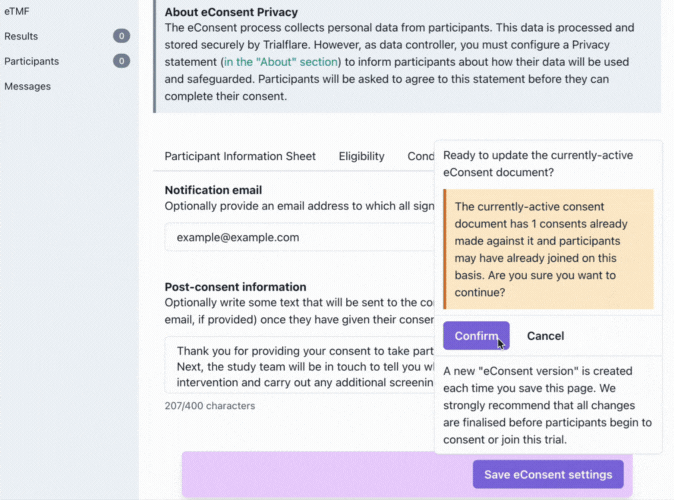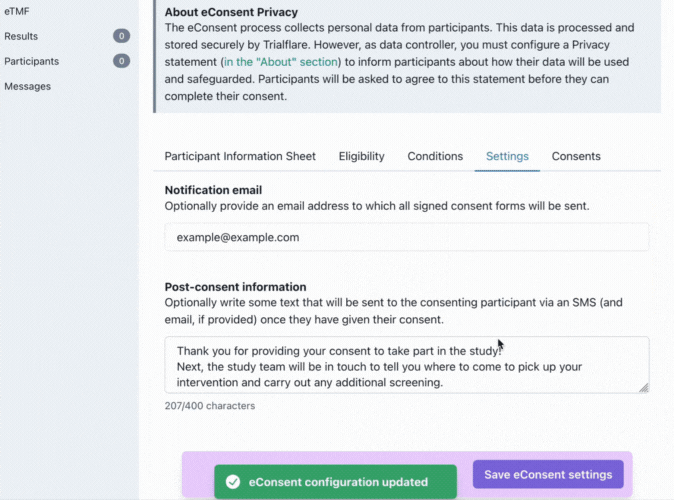
Saving your eConsent form and version control
Don't forget to click on the purple button to save your eConsent version!
We understand that sometimes things don't go according to plan and you might need to make changes to your consent document. What happens when things have changed? You will need to issue a new version for the participant to consent to. New versions which are saved replace previous ones, but the previous consents provided by participants are still logged. Participants taking part in the existing study will need to re-consent on their app! This is a little bit of extra work for the participant, but ensures that they are kept informed of any changes which were needed.
👉🏻 What does the eConsent process look like for the participant? How does it work for them?