Background
You've now created your informed consent form (ICF) for eConsent - great! But what's next? How does the participant get this information? How do they get safely onboarded and how do we bridge the consent to a specific participant?
Share your new eConsent link with participants
-
Generate a QR code or share the URL. It will be in the format:
-
Participants will review and complete this on the web, so they can use any device (desktop, smartphone, tablet) to take part.
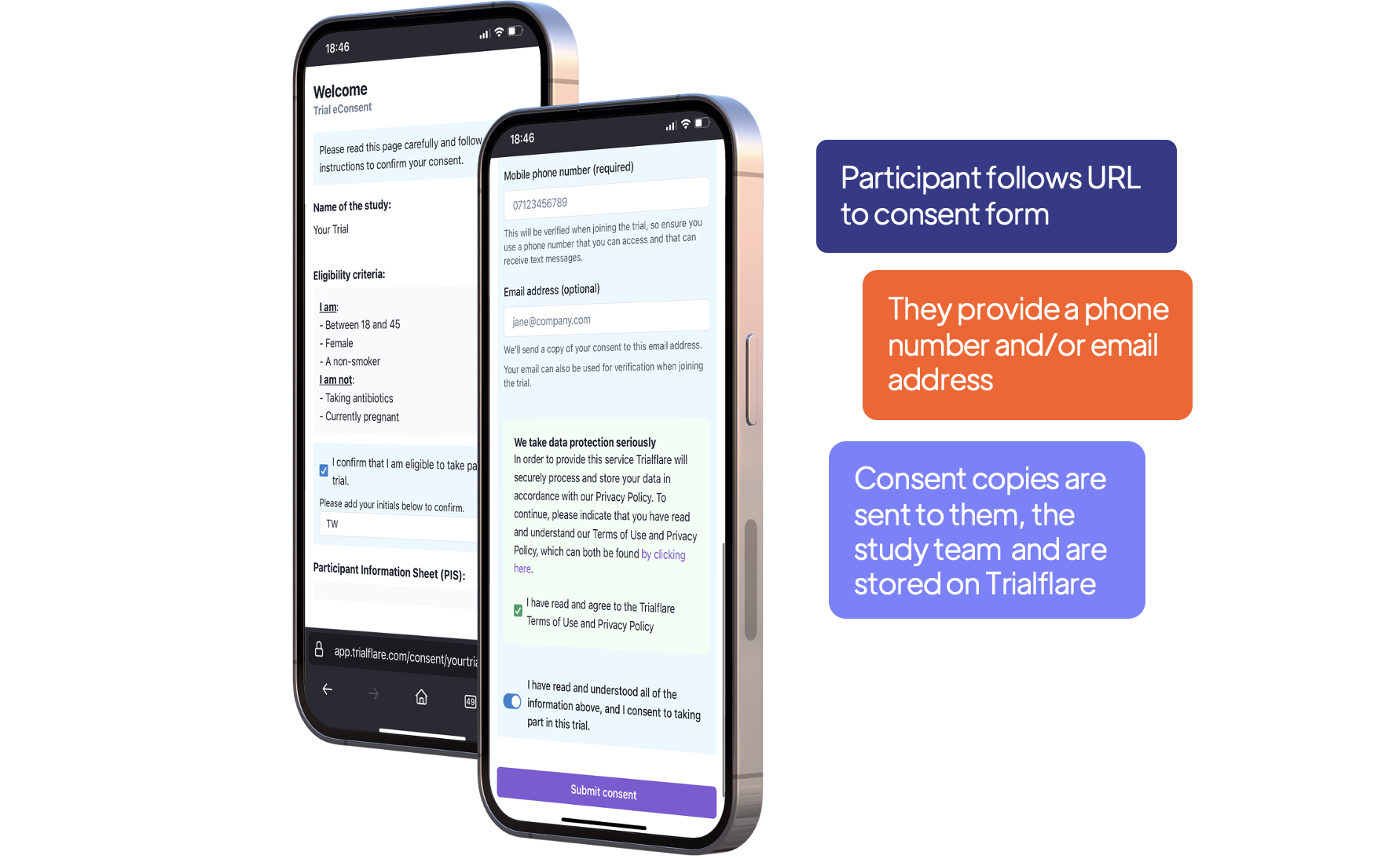
Post-consent - What happens next?
After the participant has provided consent, you'll want to know that it is really them - and tie this to some personally identifiable information (PII). In this case, we use SMS and/or email.
When the participant logs in for the first time to the study, they will be asked to enter the phone number or email address that provided the consent.
🧑🏻💻 First-time login for participants (web or smartphone app)
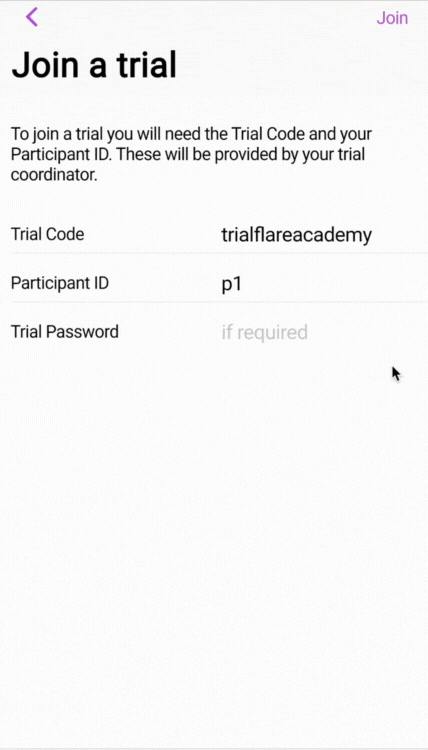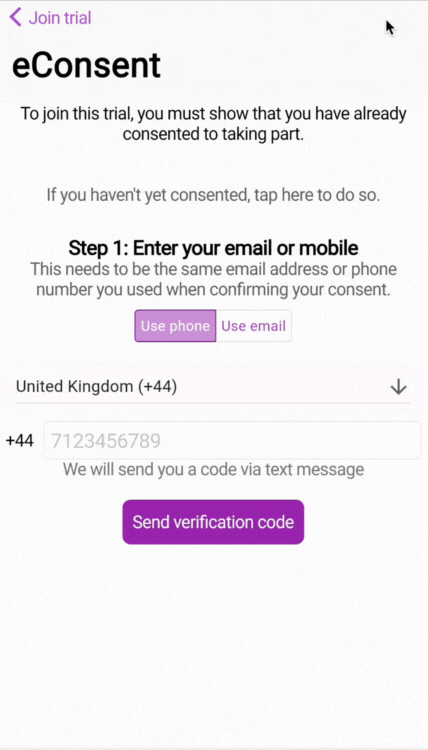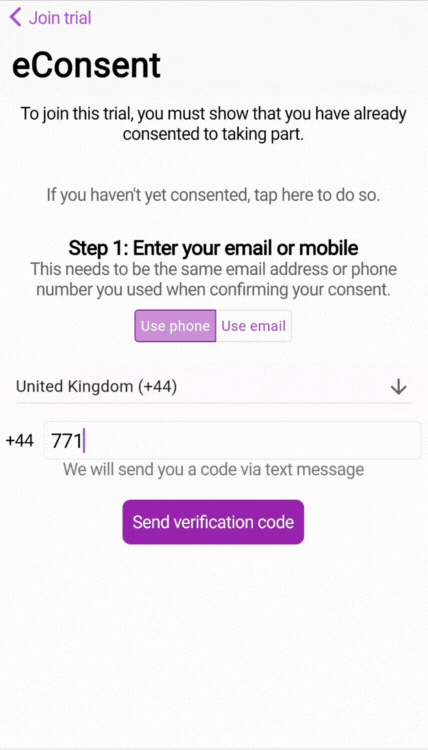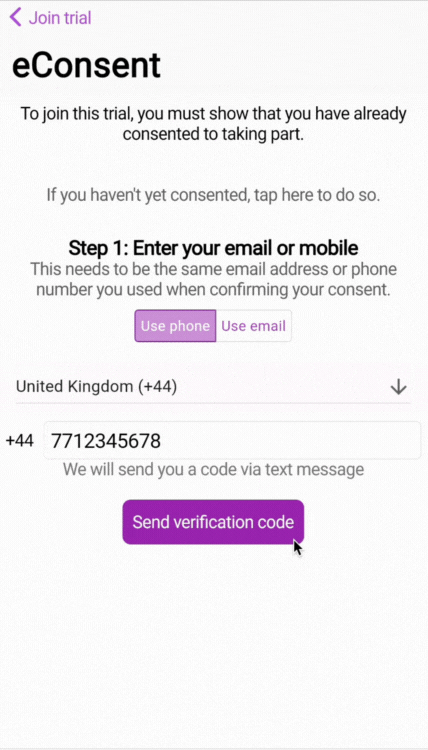
💡 IMPORTANT: You'll notice that there is a blocker on the study when eConsent is enabled. According to best practices (E.g., ICH-GCP), we don't want participants to engage with any part of the research unless they have provided consent.
By entering the phone number or email address attached to the consent, we are able to use 2-factor authentication (2FA) to verify the phone number and/or email address.
🧑🏻💻 Two-factor authentication (2FA) login

Once the phone number or email address has been validated, the participant will be able to login as normal and see any stages you wish them to complete.
Of course, if you are not conducting any ePRO / off-site submissions from participants, you won't need to do this.
What happens next? How do participants get started and what happens?